

2023-01-06
Author: Matt Kremenetsky
Progress in additive manufacturing (AM) for medication is much farther along than might be suggested by the relative scarcity of coverage on the subject in trade publications. One reason for the gap between reality and perception, perhaps, is simply that the term ‘AM’ encompasses such a wide range of technologies.
Thus, it can be quite difficult to compare the respective pace at which each market segment under the AM umbrella is proceeding. There’s enough overlap between how AM is used in, say, the aerospace and automotive sectors, that it’s a fairly straightforward process to compare the respective progress of AM in each of these market segments. Comparisons become far more difficult the more self-contained a market segment is from all the others, meaning you have to shift your perspective accordingly in order to gauge the evolution of something like additive construction (AC), or food printing.
Or AM for pharmaceuticals. Maybe more than any other market segment, the progress made in this one has to be judged according to its own criteria. For instance, one of the companies that’s made the most progress in the 3D printed medication space, Triastek (headquartered in Nanjing), was founded in 2015 — yet still hasn’t brought any of its products to market. In fact, Spritam, an epilepsy medication made by Aprecia Pharmaceuticals that received FDA approval in 2015, remains the only commercially-available 3D printed pharmaceutical in the US.
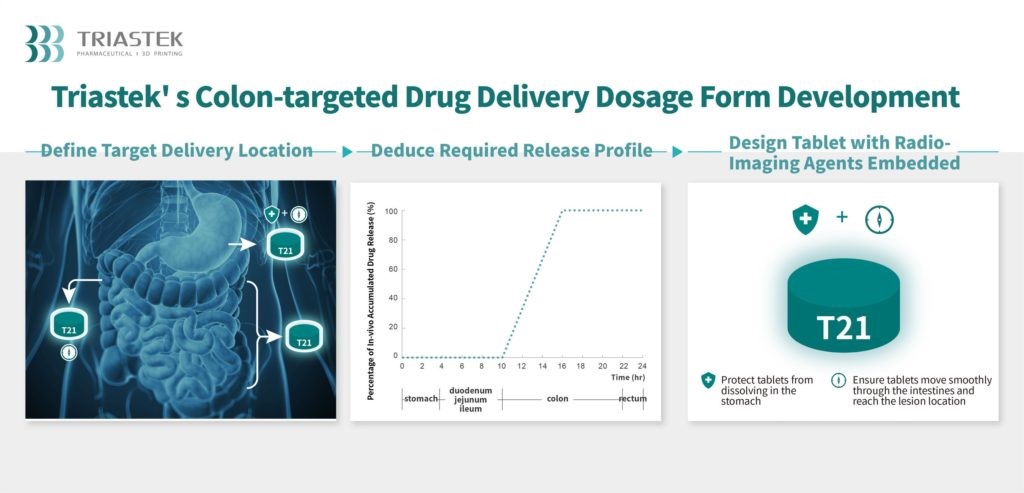
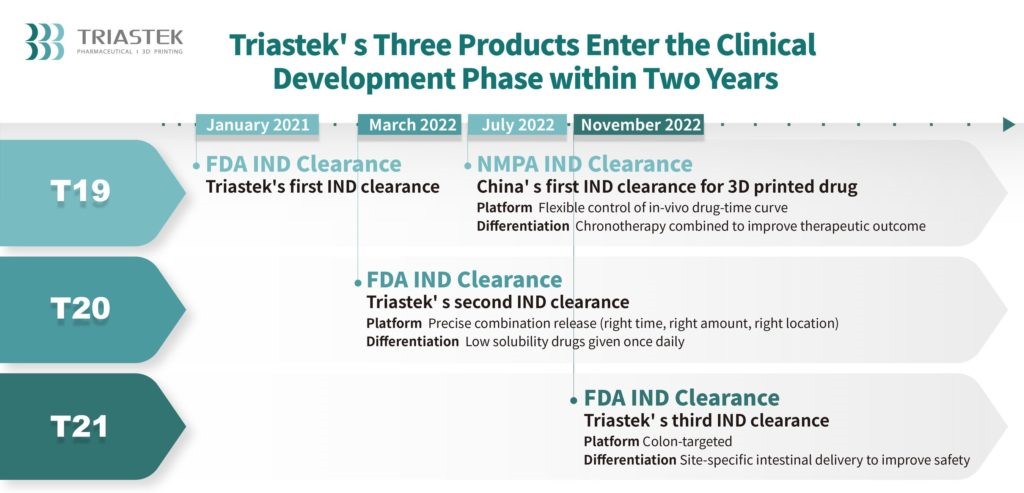
However, that says far more about the arduousness of the regulatory process involved in commercializing pharmaceuticals, than it does about the viability of AM for medication. Triastek’s progress can be gleaned from the fact that in November, 2022, it received its third Investigational New Drug (IND) clearance from the FDA in two years, for 3D printed medications produced with the company’s patented Melt-Extrusion Deposition (MED) technology. This latest clearance is for an ulcerative colitis drug, T21, which means that Triastek now has the okay to run clinical trials on it. Assuming the company successfully completes the final step in the process — New Drug Application (NDA) approval — T21 will be commercially available on the US market.
I recently interviewed Dr. Senping Cheng, Triastek’s founder and CEO, to learn more. According to Dr. Cheng, “Currently, we are conducting a study around our first product on the continuous production line, with an NDA submission in 2024 or 2025.” That drug, a rheumatoid arthritis medication called T19, is a perfect reflection of why AM for pharmaceuticals has so much potential: the ability to produce unique geometries can be harnessed to control the drug’s effects.

Dr. Senping Cheng, Triastek’s founder and CEO
“Our development process can create alternative versions of existing drugs that ultimately have functionality to better benefit patients,” said Dr. Cheng. “[T19] is designed to be taken at night, with the tablet releasing the drug in a delayed manner such that the blood concentration peaks in the early morning hours when the symptoms of pain, joint stiffness and dysfunction are most acute.” The effects of T21 are similarly augmented by 3D printing: “[T21] specifically releases medicine in the colon segment of the gastrointestinal tract, providing a localized drug effect, and mitigating potential side effects from systemic exposure [that could result] if the medicine was released elsewhere in the body.”
Earlier in 2022, global pharma giant Eli Lilly announced that it would be collaborating with Triastek, through Lilly China Innovation & Partnerships, to research potential new applications for the MED platform. It’s not clear if T21 is the first product of that collaboration, but the relative timing of both announcements certainly seems notable.
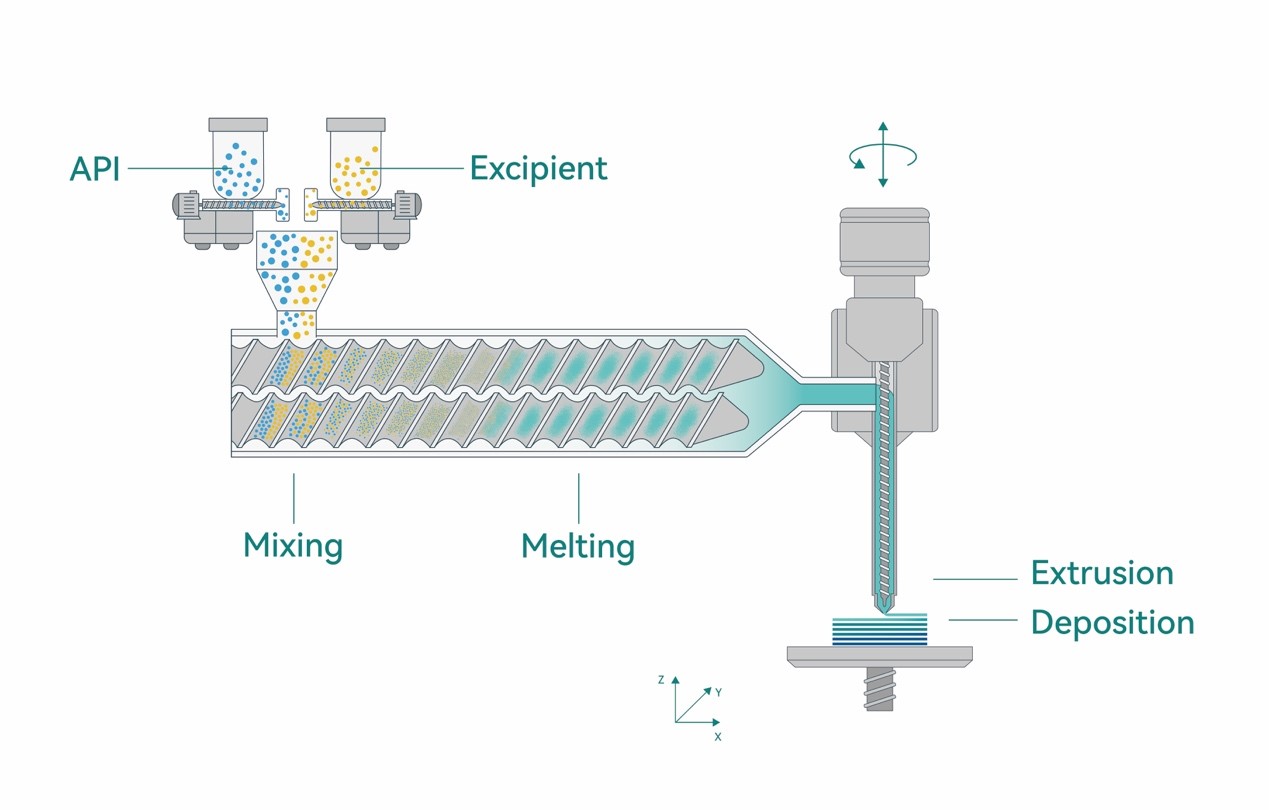
In any case, Eli Lilly’s interest in Triastek presumably stems from the MED platform’s uniqueness. Methods for 3D printing drugs typically involve a form of binder-jetting. Extrusion methods like MED, on the other hand, could make it easier for suppliers to scale up the use of AM for pharmaceuticals, since it allows manufacturers to bypass the step of preparing input materials in the print-bed.

Regarding that specific benefit, Dr. Cheng noted, “On demand continuous manufacturing is one of the key advantages of MED technology over other 3D printing technologies. …Our platform can scale out and up for continuous, clinical supply and commercial manufacturing, backed by a digital core. The agility of our technology allows us to focus on as big or as small a batch of medicine as needed for our partners, making us an ideal partner for pharmaceutical developers of varying size.”
In particular, the potential for automating pharmaceutical production that this implies could lead to a sudden upsurge in interest in 3D printed pharmaceuticals over the next few years. By the end of 2022, the mainstream news cycle had become saturated with stories revolving around shortages of critical medications, including amoxicillin, with the supply chain disruptions now even affecting over-the-counter basics like children’s ibuprofen. These stories seem to all involve labor shortages at the affected companies, meaning more automation is likely coming in the pharma industry.
And the more that extrusion 3D printing pharmaceuticals is embraced as a solution to fill supply gaps, the more likely it will be that both suppliers and customers will explore other advantages of the technology. The most exciting of those is the ability to combine active pharmaceutical ingredients (API) from multiple medications in the same dose, which, among other things, would make it easier for patients with multiple prescriptions to remember to take them on time.
As Dr. Cheng explained, “…[W]e can develop a multi-compartment prototype where multiple API’s can be printed into separate compartments, with each compartment having a unique surface area to control the release rate, and a delayed-release layer with a specific thickness to control the in vivo release onset time and location of the API. We can also use the same structure to achieve different release profiles for a single API. By doing this, we can significantly improve the solubility and bioavailability of poorly soluble APIs.”
Thus, MED technology is not only capable of facilitating an easier medication schedule for patients, but could also, even more crucially, make those same medications more effective.
The takeaway here is that the results of technological progress are asymmetrical. One market segment in the AM sector may currently look like it has the lead in realizing AM’s potential, but the sector is still in such an early stage of its history, and the relevant regulatory frameworks are so indeterminate and so scattered, that the landscape could change dramatically at any moment. Moreover, if Triastek meets its target deadlines, we’re only 1-2 years away from seeing at least one new 3D printed pharmaceutical on the market. I think it’s realistic to expect that it will be quickly followed by many others.
BD@triastek.com
苏ICP备17053162号 苏公网安备 32011502011777号
All Rights Reserved
Copyright © 2026 (TRIASTEK)
Contact Us
BD@triastek.comCOPYRIGHT © 2026 TRIASTEK. 苏ICP备17053162号
DIGITAL By VTHINK