

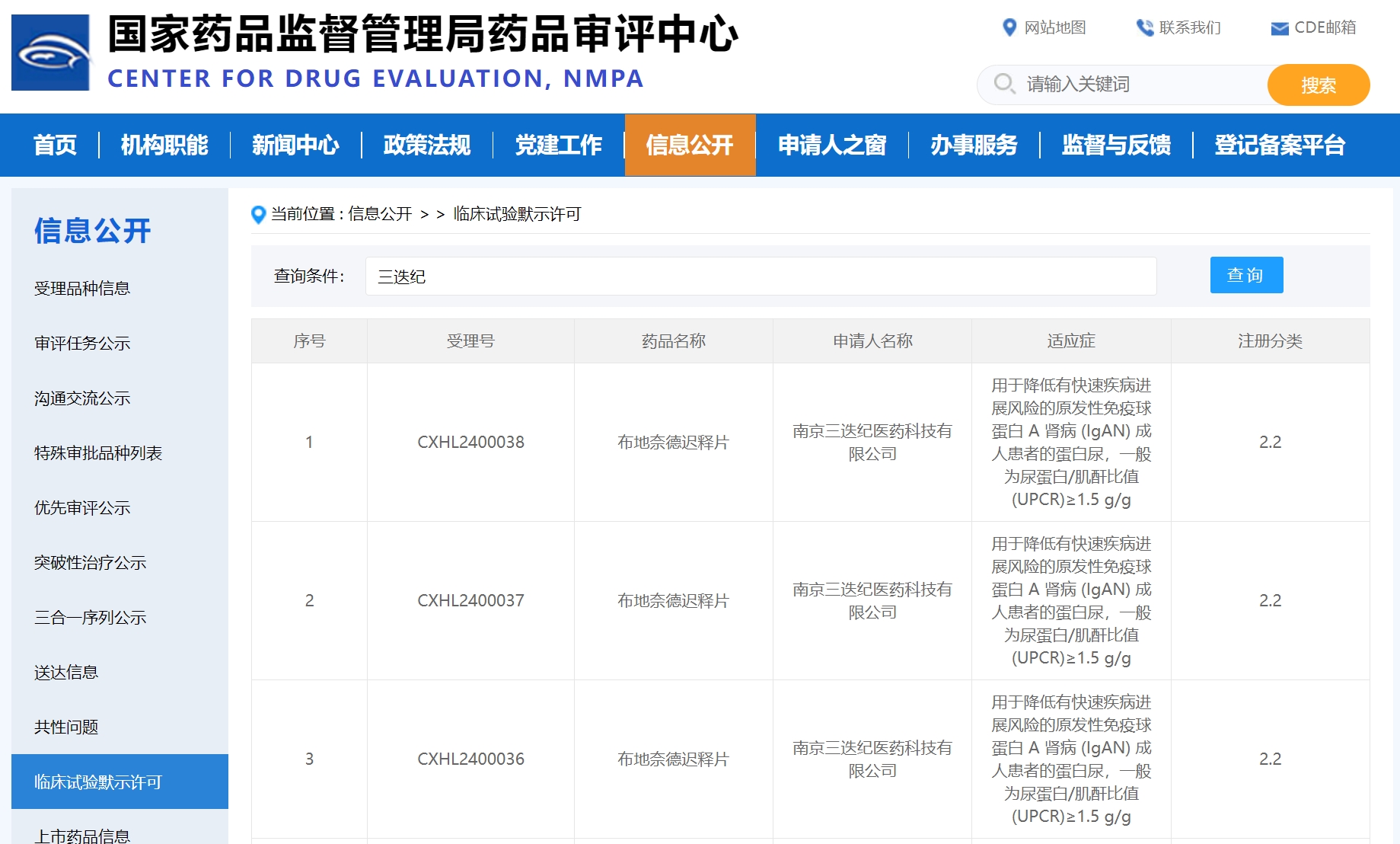
2024-03-29
Triastek's D23 budesonide-containing product has become the third 3D printed drug in China to enter clinical stage development. D23 is developed using the 3D Microstructure for Modified Release (3DμS®-MR) platform.
Currently, Triastek has five 3D printed products, T19, T20, T21, T22, and D23 that have obtained IND clearance, ranking Triastek first in the global 3D printing field for pharmaceutical development.
On March 29, Triastek announced that the Chinese National Medical Products Administration (NMPA) granted clearance to proceed with the Investigational New Drug (IND) for D23, making it the third 3D printed product to receive this designation in China.
Primary immunoglobulin A nephropathy (IgAN) is the most common primary glomerular disease and an important cause of chronic kidney disease (CKD) and end-stage renal disease (ESRD). Until recently, there were no therapies directly treating IgA nephropathy (IgAN), instead relying on supportive treatments such as controlling blood pressure and improving proteinuria, which have limited efficacy. In some cases, hormones combined with immunosuppressants have been used, but the incidence of adverse reactions is relatively high.
In December 2023, the US Food and Drug Administration (FDA) granted Tarpeyo (budesonide) full approval for the treatment of IgAN with the aim of reducing the loss of kidney function, providing the first direct treatment for IgAN patients. The Tarpeyo product utilizes a complex formulation using drug-containing pellets (3 different layers of coating) + capsule shell coating (3 different layers of coating) designed to release budesonide in a delayed fashion to the target tissue, the Peyer's lymph node in the ileum. However, due to the complexity and instability of the process, its production cost remains high, and the three-layer pellet coating process results in low drug loading. Patients receiving a typical daily dose of 16 mg need to take 4 size 1 capsules at each dose, for up to 9 months, posing a medication compliance issue.
Triastek's D23 is an improved oral budesonide delayed-release tablet via the company's Melt Extrusion Deposition (MED®) process utilizing the 3D Microstructure for Modified Release (3DμS®-MR) platform.
D23 is comprised of a multi-granule core and a delay layer to assure delivery to the target tissue before drug release. The delay layer protects the amorphous dispersion preventing early crystallization prior to reaching the target tissue. Using this unique structural design, after oral administration the drug core is accurately delivered to the Peyer's lymph nodes in the ileum where the drug-containing particles rapidly disperse and provide a sustained release budesonide resulting in delivery of budesonide to the entire target area at a high drug concentration. By acting directly on Peyer's lymph nodes in the ileum, budesonide reduces the production of IgA that lacks lactose acidification (Gd-IgA), thereby providing a direct treatment for IgAN.
Compared to the originator product, D23 can reduce the patient's medication burden and thereby improve treatment compliance through a reduction in tablet size and number. Because of the continuous production process afforded by MED 3D printing, D23's production costs are expected to be significantly reduced compared to the originator, resulting in a reduced cost to patients and thus less economic burden and improved access.
Dr. Senping Cheng, founder and CEO of Triastek, said: "D23 is developed based on the 3D Microstructure for Modified Release platform and uses simple 3D printing process MED. We believe 3D printing technology can provide an efficient development platform and high quality production process for IgA nephropathy products, and to bring more clinically valuable products to patients."
With the NMPA clearance to proceed for D23, this brings a total of five of Triastek's 3D printed drug products to the clinical development stage. This not only demonstrates the broad applications of Triastek's technology platform, but also further consolidates the company's leading position in the field of 3D printed pharmaceuticals. Currently, Triastek is actively exploring the global market through two business models, respectively "Product License-out Partnership" and "Technology Platform Partnership".
About Triastek
Global leader in 3D printing pharmaceuticals, Pioneer of new digital pharmaceutical processes
Founded in July 2015, Triastek is dedicated to the mission of "revolutionizing the pharmaceutical industry, unlocking the next generation of medicine and benefitting patients worldwide." We have created the Melt Extrusion Deposition (MED®) 3D printing pharmaceutical process, transforming the delivery, development, and production of medicines through digital product development and continuous manufacturing.
Leveraging our proprietary 3D printing pharmaceutical technology, we design and develop transformative medicines for patients. Through collaboration with global pharmaceutical companies, we tackle challenging formulation problems, accelerating the development of new drug products and improving the quality of pharmaceuticals.
Our vision is to become the world's most influential intelligent pharmaceutical enterprise.
BD@triastek.com
苏ICP备17053162号 苏公网安备 32011502011777号
All Rights Reserved
Copyright © 2026 (TRIASTEK)
Contact Us
BD@triastek.comCOPYRIGHT © 2026 TRIASTEK. 苏ICP备17053162号
DIGITAL By VTHINK