

January 2024
NMPA IND Clearance
February 2025
FDA IND Clearance
Differentiation: The specialized Bloom Structure design enables sustained drug absorption in the upper gastrointestinal tract, simplifying the dosing regimen and enhancing oral bioavailability.
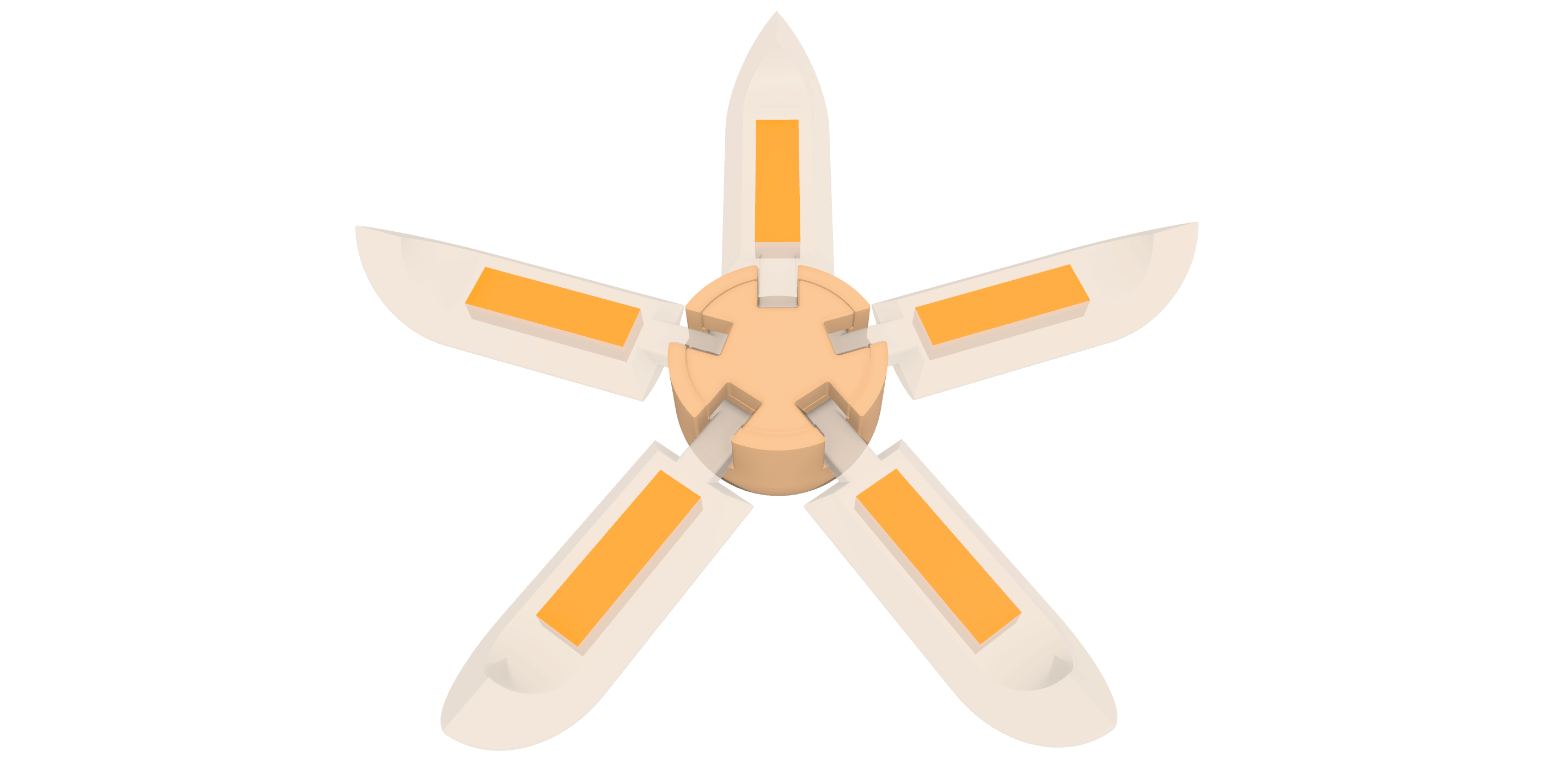
Platform: 3D Microstructure for Gastric Retention

January 2024
FDA IND Clearance
Differentiation: The specialized Bloom Structure design enables sustained drug absorption in the upper gastrointestinal tract, simplifying the dosing regimen and enhancing oral bioavailability.
Platform: 3D Microstructure for Gastric Retention
March 2024
NMPA IND Clearance
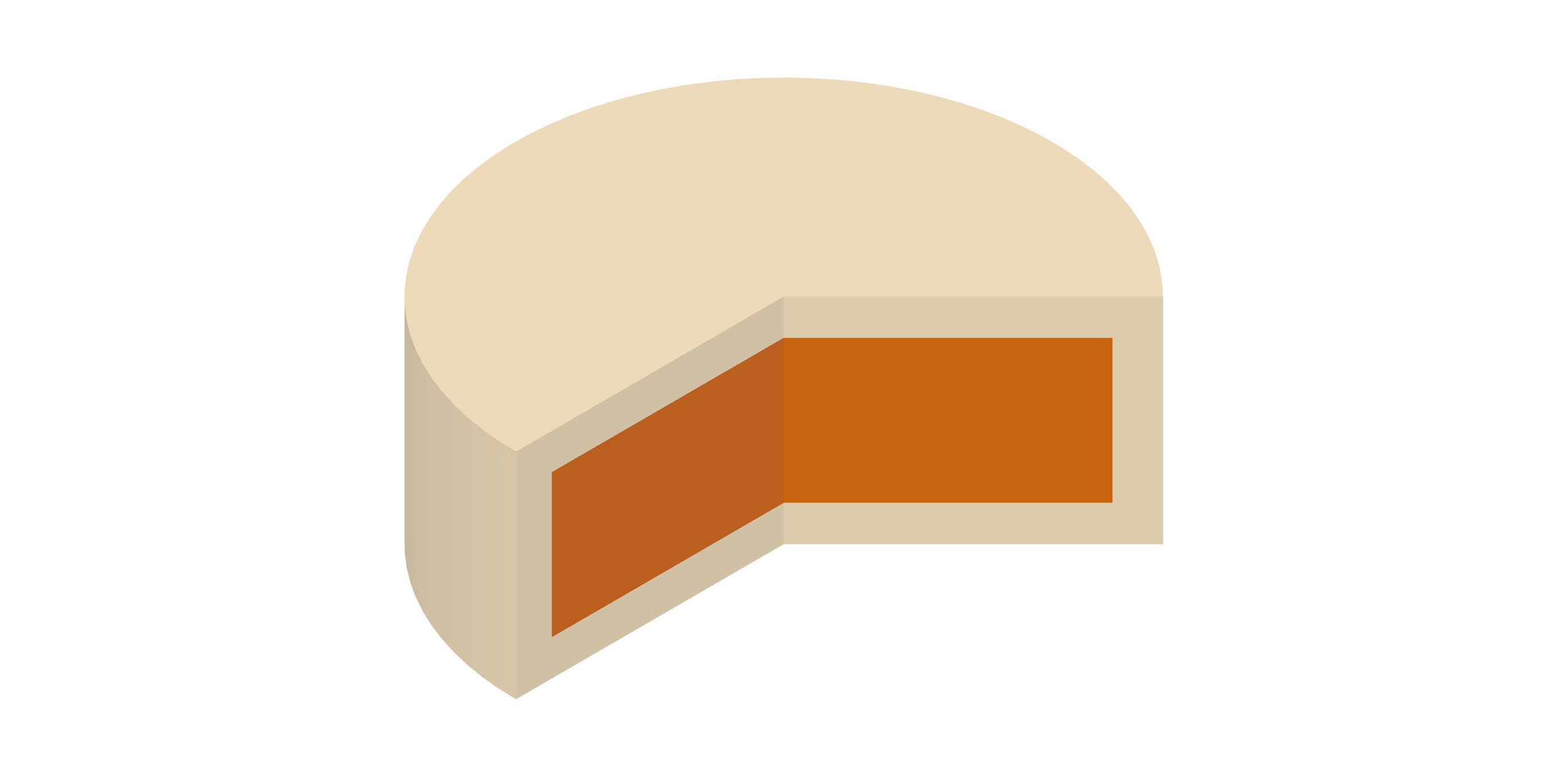
Differentiation: Allowing for precise targeting of drug release and delivery to the ileum ensures that the API reaches the ileal area of the intestine, where the disease originates and where API is most beneficial.
Platform: 3D Microstructure for Intestine Targeting
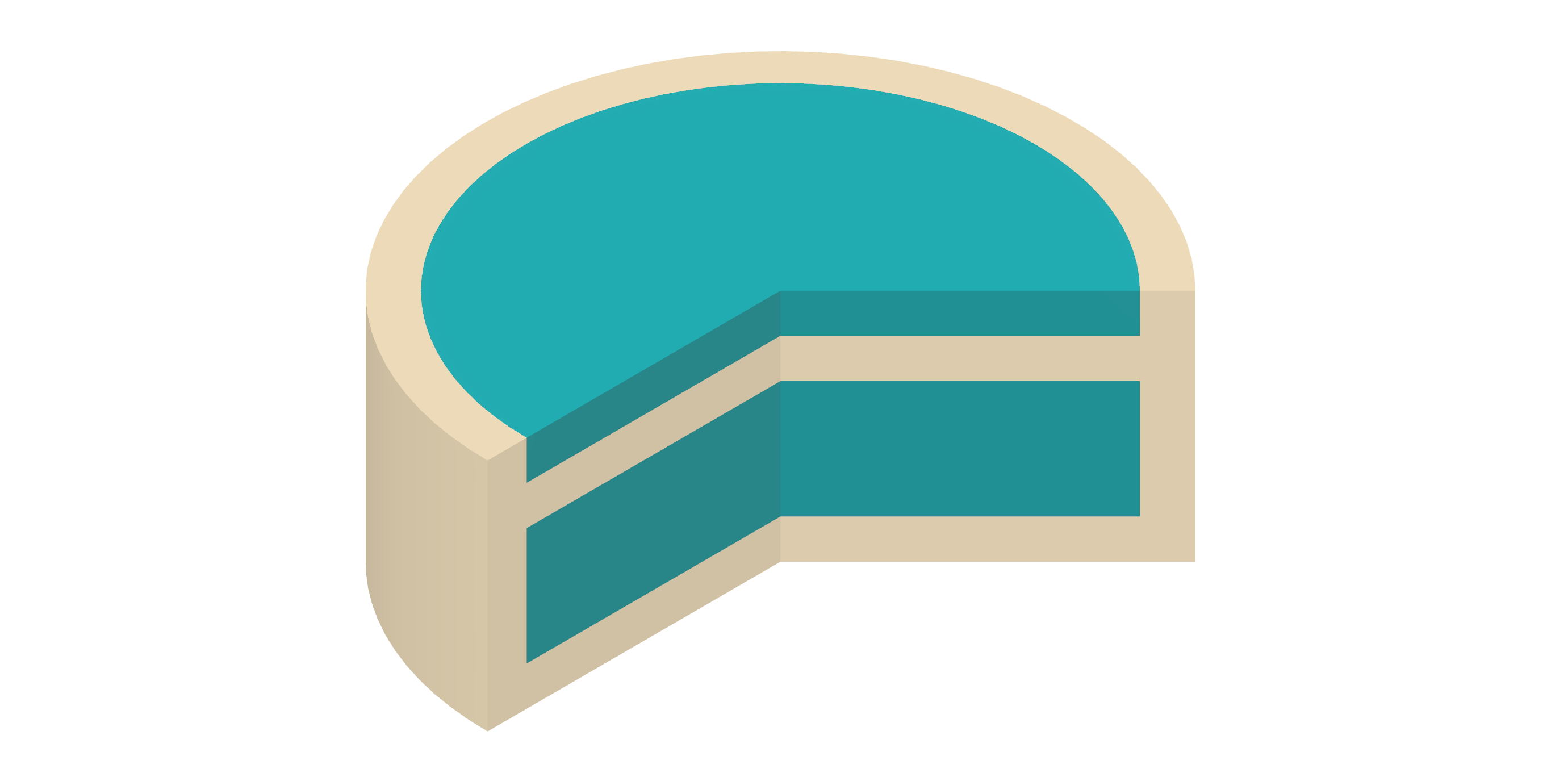
Differentiation: The dual-release tablet formulation, with an additional secondary drug release phase, holds promise for maintaining rapid sleep-onset efficacy while effectively preventing early-morning awakenings in insomnia patients and ameliorating comorbid symptoms such as sleep maintenance insomnia.
Platform: 3D Microstructure for Modified Release
January 2024
NMPA IND Clearance
February 2025
FDA IND Clearance
Differentiation: The specialized Bloom Structure design enables sustained drug absorption in the upper gastrointestinal tract, simplifying the dosing regimen and enhancing oral bioavailability.
Platform: 3D Microstructure for Gastric Retention

January 2024
FDA IND Clearance
Differentiation: The specialized Bloom Structure design enables sustained drug absorption in the upper gastrointestinal tract, simplifying the dosing regimen and enhancing oral bioavailability.
Platform: 3D Microstructure for Gastric Retention

March 2024
NMPA IND Clearance
Differentiation: Allowing for precise targeting of drug release and delivery to the ileum ensures that the API reaches the ileal area of the intestine, where the disease originates and where API is most beneficial.
Platform: 3D Microstructure for Intestine Targeting

Differentiation: The dual-release tablet formulation, with an additional secondary drug release phase, holds promise for maintaining rapid sleep-onset efficacy while effectively preventing early-morning awakenings in insomnia patients and ameliorating comorbid symptoms such as sleep maintenance insomnia.
Platform: 3D Microstructure for Modified Release

RA: Rheumatoid Arthritis; PsA: Psoriatic Arthritis; AS: Ankylosing Spondylitis; AF: Atrial Fibrillation; VTE: Venous Thromboembolism; UC: Ulcerative Colitis; PAH: Pulmonary Arterial Hypertension; IgAN: IgA nephropathy
BD@triastek.com
苏ICP备17053162号 苏公网安备 32011502011777号
All Rights Reserved
Copyright © 2026 (TRIASTEK)
Contact Us
BD@triastek.comCOPYRIGHT © 2026 TRIASTEK. 苏ICP备17053162号
DIGITAL By VTHINK